标准电极电势
维基媒体列表条目
标准电极电势是可逆电极在标准状态及平衡态时的电势,也就是标准态时的平衡电势,记作、或,上标表示标准态。标准状态的溶质活度每公升1莫爾,气体压强10萬Pa,温度一般為298K。虽然电池的电动势可以直接测定,但单一可逆电极的标准电极电势却只有相对值没有绝对值,而且随温度、浓度和压强而变。电极电势的基准是标准氢电极:标准状态的H⁺/H₂电极电势定为0V,即,其他电极电势的值在此基础上获得。当某半电池和标准氢电池电极相连时为负极,该半电池的半反应的标准电极电势为负值,且绝对值与电池电动势相等;若为正极则相反。这样求得的值称作还原电势,总反应的标准电极电势也就是两个半反应标准电极电势的差,Eo為正时反应自发。任何温度下标准氢电极的标准电极电势值都为0,但其他电极电势值会受到温度影响。以Ni/NiO电极为例,它可以用作高温伪参比电极,电极电势在0至400°C大致符合以下公式:[1]
- ,T为温度
标准电极电势可以实验或热力学计算获得。,标准氢电极的反应自由能是零,水合氢离子和水合电子的标准生成自由能也是零,这样便可计算众多无法用实验测得的标准电极电势值(如氟气)。标准电极电势沒有加成性,要想求相加得到的第三反应的标准电极电势,还需要借助上述公式来求解。标准电极电势不随半反应的方向和计量系数改变,但它受到浓度和反应物形态影响。非标态的电极电势值可以能斯特方程求得。标准电极电势值只是热力学数据,不可用于预测反应速率和其他动力学性质,而且它的值在水溶液体系中测定,不可用于其他溶剂或高温时的反应。
- 能斯特方程:
标准电极电势有很大的实用价值,可用来判断氧化剂与还原剂的相对强弱,判断氧化还原反应的方向,计算原电池的电动势、反应自由能、平衡常数,计算其他半反应的标准电极电势,等等。将半反应按电极电势由低到高排序,可以得到标准电极电势表,可十分简明判断氧还反应的方向。
標準電極電勢表编辑
表中電極電勢以以下條件測得((s):固體;(l):液體;(g):氣體;(aq):水溶液;(Hg):汞齊):
- 參比電極:標準氫電極,所有離子的數據都在水溶液測得[2][3][4][5][6][7][8][9][10]
- 活性度:統一為純固體、純液體或水溶液
- 溫度:298.15K/25攝氏度
- 水溶液或汞齊之有效離子濃度:每公升1摩
- 氣體反應物分壓:1大氣壓/101325帕
单击頂栏箭咀可将数据按元素符号、反應物、產物或标准电极电势值排序。
| 元素 | 氧化劑 | 半反應 | 還原劑 | E°/伏 | 來源 |
|---|---|---|---|---|---|
| 鋇 | Ba⁺+e⁻ | ⇌ | Ba(s) | −4.38 | [2][4][11] |
| 鍶 | Sr⁺+e⁻ | ⇌ | Sr(s) | −4.10 | [12][2][4][13] |
| 鈣 | Ca⁺+e⁻ | ⇌ | Ca(s) | −3.8 | [12][2][4][13] |
| 釷 | Th⁴⁺+e⁻ | ⇌ | Th³⁺ | −3.6 | [14] |
| 鐠 | Pr³⁺+e⁻ | ⇌ | Pr²⁺ | −3.1 | [15] |
| 氮 | 3N₂(g)+2H⁺+2e⁻ | ⇌ | 2HN₃(aq) | −3.09 | [7] |
| 锂 | Li⁺+e⁻ | ⇌ | Li(s) | −3.0401 | [6] |
| 氮 | N₂(g)+4H₂O+2e⁻ | ⇌ | 2NH₂OH(aq)+2OH⁻ | −3.04 | [7] |
| 铯 | Cs⁺+e⁻ | ⇌ | Cs(s) | −3.026 | [6] |
| 鈣 | Ca(OH)₂(s)+2e⁻ | ⇌ | Ca(s)+2OH⁻ | −3.02 | [12] |
| 鉺 | Er³⁺+e⁻ | ⇌ | Er²⁺ | −3.0 | [16] |
| 鋇 | Ba(OH)₂(s)+2e⁻ | ⇌ | Ba(s)+2OH⁻ | −2.99 | [12] |
| 铷 | Rb⁺+e⁻ | ⇌ | Rb(s) | −2.98 | [5] |
| 鎂 | Mg⁺+e⁻ | ⇌ | Mg(s) | −2.93 | [11] |
| 钾 | K⁺+e⁻ | ⇌ | K(s) | −2.92 | [6] |
| 钡 | Ba²⁺+2e⁻ | ⇌ | Ba(s) | −2.912 | [6] |
| 镧 | La(OH)₃(s)+3e⁻ | ⇌ | La(s)+3OH⁻ | −2.90 | [17] |
| 鈁 | Fr⁺+e⁻ | ⇌ | Fr(s) | −2.9 | [12] |
| 锶 | Sr²⁺+2e⁻ | ⇌ | Sr(s) | −2.899 | [6] |
| 鍶 | Sr(OH)₂(s)+2e⁻ | ⇌ | Sr(s)+2OH⁻ | −2.88 | [12] |
| 钙 | Ca²⁺+2e⁻ | ⇌ | Ca(s) | −2.868 | [6] |
| 氮(銨) | NH₄⁺+e⁻ | ⇌ | NH₄• | −2.85 | |
| 碳(碳鋰) | Li⁺+C₆(s)+e⁻ | ⇌ | LiC₆(s) | −2.84 | [18] |
| 铕 | Eu²⁺+2e⁻ | ⇌ | Eu(s) | −2.812 | [6] |
| 镭 | Ra²⁺+2e⁻ | ⇌ | Ra(s) | −2.8 | [6] |
| 鈥 | Ho³⁺+e⁻ | ⇌ | Ho²⁺ | −2.8 | [13] |
| 鉳 | Bk³⁺+e⁻ | ⇌ | Bk²⁺ | −2.8 | [13] |
| 鐿 | Yb²⁺+2e⁻ | ⇌ | Yb(s) | −2.76 | [12][2] |
| 钠 | Na⁺+e⁻ | ⇌ | Na(s) | −2.71 | [6][10] |
| 釹 | Nd³⁺+e⁻ | ⇌ | Nd²⁺ | −2.7 | [13] |
| 鎂 | Mg(OH)₂+2e⁻ | ⇌ | Mg(s)+2OH⁻ | −2.69 | [13] |
| 釤 | Sm²⁺+2e⁻ | ⇌ | Sm(s) | −2.68 | [12][2] |
| 鈹 | Be₂O₃²⁻+3H₂O+4e⁻ | ⇌ | 2Be(s)+6OH⁻ | −2.63 | [13] |
| Pm | Pm³⁺+e⁻ | ⇌ | Pm²⁺ | −2.6 | [13] |
| Dy | Dy³⁺+e⁻ | ⇌ | Dy²⁺ | −2.6 | [13] |
| 鍩 | No²⁺+2e⁻ | ⇌ | No(s) | −2.50 | [12] |
| 鉿 | HfO(OH)₂(s)+H₂O+4e⁻ | ⇌ | Hf(s)+4OH⁻ | −2.50 | [12] |
| 釷 | Th(OH)₄(s)+4e⁻ | ⇌ | Th(s)+4OH⁻ | −2.48 | [12] |
| 鍆 | Md²⁺+2e⁻ | ⇌ | Md(s) | −2.40 | [12] |
| Tm | Tm²⁺+2e⁻ | ⇌ | Tm(s) | −2.4 | [13] |
| 鑭 | La³⁺+3e⁻ | ⇌ | La(s) | −2.379 | [6] |
| 釔 | Y³⁺+3e⁻ | ⇌ | Y(s) | −2.372 | [6] |
| 镁 | Mg²⁺+2e⁻ | ⇌ | Mg(s) | −2.372 | [6] |
| 鋯 | ZrO(OH)₂(s)+H₂O+4e⁻ | ⇌ | Zr(s)+4OH⁻ | −2.36 | [6] |
| 鐠 | Pr³⁺+3e⁻ | ⇌ | Pr(s) | −2.353 | [12] |
| 鈰 | Ce³⁺+3e⁻ | ⇌ | Ce(s) | −2.336 | [12] |
| 鉺 | Er³⁺+3e⁻ | ⇌ | Er(s) | −2.331 | [12] |
| 鈥 | Ho³⁺+3e⁻ | ⇌ | Ho(s) | −2.33 | [12] |
| 鋁 | Al(OH)₄⁻+3e⁻ | ⇌ | Al(s)+4OH⁻ | −2.33 | |
| 釹 | Nd³⁺+3e⁻ | ⇌ | Nd(s) | −2.323 | [13] |
| Tm | Tm³⁺+3e⁻ | ⇌ | Tm(s) | −2.319 | [13] |
| 鋁 | Al(OH)₃(s)+3e⁻ | ⇌ | Al(s)+3OH⁻ | −2.31 | |
| 釤 | Sm³⁺+3e⁻ | ⇌ | Sm(s) | −2.304 | [13] |
| Fm | Fm²⁺+2e⁻ | ⇌ | Fm | −2.3 | [13] |
| Am | Am³⁺+e⁻ | ⇌ | Am²⁺ | −2.3 | [13] |
| Dy | Dy³⁺+3e⁻ | ⇌ | Dy(s) | −2.295 | [13] |
| Lu | Lu³⁺+3e⁻ | ⇌ | Lu(s) | −2.28 | [13] |
| 鋱 | Tb³⁺+3e⁻ | ⇌ | Tb(s) | −2.28 | |
| 釓 | Gd³⁺+3e⁻ | ⇌ | Gd(s) | −2.279 | [13] |
| 氢 | H₂(g)+2e⁻ | ⇌ | 2H⁻ | −2.25 | |
| Es | Es²⁺+2e⁻ | ⇌ | Es(s) | −2.23 | [13] |
| Pm | Pm²⁺+2e⁻ | ⇌ | Pm(s) | −2.2 | [13] |
| Tm | Tm³⁺+e⁻ | ⇌ | Tm²⁺ | −2.2 | [13] |
| Dy | Dy²⁺+2e⁻ | ⇌ | Dy(s) | −2.2 | [13] |
| 锕 | Ac³⁺+3e⁻ | ⇌ | Ac(s) | −2.20 | |
| Yb | Yb³⁺+3e⁻ | ⇌ | Yb(s) | −2.19 | [13] |
| 鈹 | Be⁺+e⁻ | ⇌ | Be(s) | −2.12 | [11] |
| 鉲 | Cf²⁺+2e⁻ | ⇌ | Cf(s) | −2.12 | [12] |
| 釹 | Nd²⁺+2e⁻ | ⇌ | Nd(s) | −2.1 | [13] |
| 鈥 | Ho²⁺+2e⁻ | ⇌ | Ho(s) | −2.1 | [13] |
| 鈧 | Sc³⁺+3e⁻ | ⇌ | Sc(s) | −2.077 | [19] |
| 鋁 | AlF₆³⁻+3e⁻ | ⇌ | Al(s)+6F⁻ | −2.069 | [13] |
| 鋂 | Am³⁺+3e⁻ | ⇌ | Am(s) | −2.048 | [12] |
| Cm | Cm³⁺+3e⁻ | ⇌ | Cm(s) | −2.04 | [13] |
| 鈈 | Pu³⁺+3e⁻ | ⇌ | Pu(s) | −2.031 | [13] |
| Pr | Pr²⁺+2e⁻ | ⇌ | Pr(s) | −2 | [13] |
| 鉺 | Er²⁺+2e⁻ | ⇌ | Er(s) | −2 | [13] |
| 銪 | Eu³⁺+3e⁻ | ⇌ | Eu(s) | −1.991 | [13] |
| Lr | Lr³⁺+3e⁻ | ⇌ | Lr | −1.96 | [13] |
| 鉲 | Cf³⁺+3e⁻ | ⇌ | Cf(s) | −1.94 | [12] |
| 钙 | Ca²⁺+e⁻ | ⇌ | Ca⁺ | −1.936 | [6][12] |
| Es | Es³⁺+3e⁻ | ⇌ | Es(s) | −1.91 | [13] |
| Pa | Pa⁴⁺+e⁻ | ⇌ | Pa³⁺ | −1.9 | [13] |
| 鋂 | Am²⁺+2e⁻ | ⇌ | Am(s) | −1.9 | [12] |
| 釷 | Th⁴⁺+4e⁻ | ⇌ | Th(s) | −1.899 | [13] |
| 鐨 | Fm³⁺+3e⁻ | ⇌ | Fm(s) | −1.89 | [12] |
| Np | Np³⁺+3e⁻ | ⇌ | Np(s) | −1.856 | [13] |
| 铍 | Be²⁺+2e⁻ | ⇌ | Be(s) | −1.85 | |
| 磷 | H₂PO₂⁻+e⁻ | ⇌ | P(s)+2OH⁻ | −1.82 | [13] |
| 鍶 | Sr²⁺+2e⁻ | ⇌ | Sr(Hg) | −1.793 | [20] |
| 硼 | H₂BO₃⁻+H₂O+3e⁻ | ⇌ | B(s)+4OH⁻ | −1.79 | [21] |
| 釷 | ThO₂+4H⁺+4e⁻ | ⇌ | Th(s)+2H₂O | −1.789 | [22] |
| 鉿 | HfO²⁺+2H⁺+4e⁻ | ⇌ | Hf(s)+H₂O | −1.724 | [23] |
| 磷 | HPO₃²⁻+2H₂O+3e⁻ | ⇌ | P(s)+5OH⁻ | −1.71 | [24] |
| 矽 | SiO₃²⁻+3H₂O+4e⁻ | ⇌ | Si(s)+6OH⁻ | −1.697 | [24] |
| 鑪 | Rf⁴⁺+4e⁻ | ⇌ | Rf(s) | −1.67 | [25] |
| 铀 | U³⁺+3e⁻ | ⇌ | U(s) | −1.66 | [8] |
| 铝 | Al³⁺+3e⁻ | ⇌ | Al(s) | −1.66 | [10] |
| 钛 | Ti²⁺+2e⁻ | ⇌ | Ti(s) | −1.63 | [10] |
| 鉳 | Bk²⁺+2e⁻ | ⇌ | Bk(s) | −1.6 | [12] |
| 锆 | ZrO₂(s)+4H⁺+4e⁻ | ⇌ | Zr(s)+2H₂O | −1.553 | [6] |
| 鉿 | Hf⁴⁺+4e⁻ | ⇌ | Hf(s) | −1.55 | [12] |
| 锆 | Zr⁴⁺+4e⁻ | ⇌ | Zr(s) | −1.45 | [6] |
| 鈦 | Ti³⁺+3e⁻ | ⇌ | Ti(s) | −1.37 | [26] |
| 鈦 | TiO(s)+2H⁺+2e⁻ | ⇌ | Ti(s)+H₂O | −1.31 | |
| 碳 | C⁴⁺+4e⁻ | ⇌ | C | −1.3 | [27] |
| 鈦 | Ti₂O₃(s)+2H⁺+2e⁻ | ⇌ | 2TiO(s)+H₂O | −1.23 | |
| 鋅 | Zn(OH)₄²⁻+2e⁻ | ⇌ | Zn(s)+4OH⁻ | −1.199 | [28] |
| 錳 | Mn²⁺+2e⁻ | ⇌ | Mn(s) | −1.185 | [28] |
| 鐵 | Fe(CN)₆⁴⁻+6H⁺+2e⁻ | ⇌ | Fe(s)+6HCN(aq) | −1.16 | [29] |
| 釩 | V²⁺+2e⁻ | ⇌ | V(s) | −1.175 | [3] |
| 碲 | Te(s)+2e⁻ | ⇌ | Te²⁻ | −1.143 | [3] |
| 鈮 | Nb³⁺+3e⁻ | ⇌ | Nb(s) | −1.099 | |
| 錫 | Sn(s)+4H⁺+4e⁻ | ⇌ | SnH₄(g) | −1.07 | |
| 銦 | In(OH)₃(s)+3e⁻ | ⇌ | In(s)+3OH⁻ | −0.99 | [12] |
| 矽 | SiO₂(s)+4H⁺+4e⁻ | ⇌ | Si(s)+2H₂O | −0.91 | |
| 硼 | B(OH)₃(aq)+3H⁺+3e⁻ | ⇌ | B(s)+3H₂O | −0.89 | |
| 鐵 | Fe(OH)₂(s)+2e⁻ | ⇌ | Fe(s)+2OH⁻ | −0.89 | [29] |
| 鐵 | Fe₂O₃(s)+3H₂O+2e⁻ | ⇌ | 2Fe(OH)₂(s)+2OH⁻ | −0.86 | [29] |
| 鈦 | TiO²⁺+2H⁺+4e⁻ | ⇌ | Ti(s)+H₂O | −0.86 | |
| 水 | 2H₂O+2e⁻ | ⇌ | H₂(g)+2OH⁻ | −0.8277 | [6] |
| 鉍 | Bi(s)+3H⁺+3e⁻ | ⇌ | BiH₃ | −0.8 | [28] |
| 锌 | Zn²⁺+2e⁻ | ⇌ | Zn(Hg) | −0.7628 | [6] |
| 锌 | Zn²⁺+2e⁻ | ⇌ | Zn(s) | −0.7618 | [6] |
| 钽 | Ta₂O₅(s)+10H⁺+10e⁻ | ⇌ | 2Ta(s)+5H₂O | −0.75 | |
| 铬 | Cr³⁺+3e⁻ | ⇌ | Cr(s) | −0.74 | |
| 鎳 | Ni(OH)₂(s)+2e⁻ | ⇌ | Ni(s)+2OH⁻ | −0.72 | [30] |
| 銀 | Ag₂S(s)+2e⁻ | ⇌ | 2Ag(s)+S²⁻(aq) | −0.69 | |
| 金(金氰) | Au(CN)₂⁻+e⁻ | ⇌ | Au(s)+2CN⁻ | −0.60 | |
| 鉭 | Ta³⁺+3e⁻ | ⇌ | Ta(s) | −0.6 | |
| 鉛 | PbO(s)+H₂O+2e⁻ | ⇌ | Pb(s)+2OH⁻ | −0.58 | |
| 鈦 | 2TiO₂(s)+2H⁺+2e⁻ | ⇌ | Ti₂O₃(s)+H₂O | −0.56 | |
| 镓 | Ga³⁺+3e⁻ | ⇌ | Ga(s) | −0.53 | |
| 鈾 | U⁴⁺+e⁻ | ⇌ | U³⁺ | −0.52 | [8] |
| 磷 | H₃PO₂(aq)+H⁺+e⁻ | ⇌ | P(白)[31]+2H₂O | −0.508 | [6] |
| 磷 | H₃PO₃(aq)+2H⁺+2e⁻ | ⇌ | H₃PO₂(aq)+H₂O | −0.499 | [6] |
| 鎳 | NiO₂(s)+2H₂O+2e⁻ | ⇌ | Ni(OH)₂(s)+2OH⁻ | −0.49 | [32] |
| 磷 | H₃PO₃(aq)+3H⁺+3e⁻ | ⇌ | P(红)[31]+3H₂O | −0.454 | [6] |
| 銅 | Cu(CN)₂⁻+e⁻ | ⇌ | Cu(s)+2CN⁻ | −0.44 | [33] |
| 铁 | Fe²⁺+2e⁻ | ⇌ | Fe(s) | −0.44 | [10] |
| 碳 | 2CO₂(g)+2H⁺+2e⁻ | ⇌ | (HO₂C)₂(aq) | −0.43 | |
| 鉻 | Cr³⁺+e⁻ | ⇌ | Cr²⁺ | −0.42 | |
| 镉 | Cd²⁺+2e⁻ | ⇌ | Cd(s) | −0.40 | [10] |
| 硒 | SeO₃²⁻+4e⁻+3H₂O | ⇌ | Se+6OH⁻ | −0.37 | [34] |
| 锗 | GeO₂(s)+2H⁺+2e⁻ | ⇌ | GeO(s)+H₂O | −0.37 | |
| 铜 | Cu₂O(s)+H₂O+2e⁻ | ⇌ | 2Cu(s)+2OH⁻ | −0.360 | [6] |
| 铅 | PbSO₄(s)+2e⁻ | ⇌ | Pb(s)+SO₄²⁻ | −0.3588 | [6] |
| 铅 | PbSO₄(s)+2e⁻ | ⇌ | Pb(Hg)+SO₄²⁻ | −0.3505 | [6] |
| 铕 | Eu³⁺+e⁻ | ⇌ | Eu²⁺ | −0.35 | [8] |
| 铟 | In³⁺+3e⁻ | ⇌ | In(s) | −0.34 | [3] |
| 铊 | Tl⁺+e⁻ | ⇌ | Tl(s) | −0.34 | [3] |
| 酶 | NAD(P)⁺+H⁺+2e⁻ | ⇌ | NAD(P)H | −0.32 | [35] |
| 硼 | B³⁺+3e⁻ | ⇌ | B(s) | −0.31 | |
| 锗 | Ge(s)+4H⁺+4e⁻ | ⇌ | GeH₄(g) | −0.29 | |
| 钴 | Co²⁺+2e⁻ | ⇌ | Co(s) | −0.28 | [6] |
| 磷 | H₃PO₄(aq)+2H⁺+2e⁻ | ⇌ | H₃PO₃(aq)+H₂O | −0.276 | [6] |
| 釩 | V³⁺+e⁻ | ⇌ | V²⁺ | −0.26 | [10] |
| 镍 | Ni²⁺+2e⁻ | ⇌ | Ni(s) | −0.25 | |
| 砷 | As(s)+3H⁺+3e⁻ | ⇌ | AsH₃(g) | −0.23 | [3] |
| 鎵 | Ga⁺+e⁻ | ⇌ | Ga(s) | −0.2 | [36] |
| 银 | AgI(s)+e⁻ | ⇌ | Ag(s)+I⁻ | −0.15224 | [28] |
| 钼 | MoO₂(s)+4H⁺+4e⁻ | ⇌ | Mo(s)+2H₂O | −0.15 | |
| 硅 | Si(s)+4H⁺+4e⁻ | ⇌ | SiH₄(g) | −0.14 | |
| 錫 | Sn²⁺+2e⁻ | ⇌ | Sn(s) | −0.13 | |
| 氧 | O₂(g)+H⁺+e⁻ | ⇌ | HO₂•(aq) | −0.13 | |
| 铅 | Pb²⁺+2e⁻ | ⇌ | Pb(s) | −0.13 | [10] |
| 钨 | WO₂(s)+4H⁺+4e⁻ | ⇌ | W(s)+2H₂O | −0.12 | |
| 磷 | P(红)+3H⁺+3e⁻ | ⇌ | PH₃(g) | −0.111 | [6] |
| 碳 | CO₂(g)+2H⁺+2e⁻ | ⇌ | HCO₂H(aq) | −0.11 | |
| 硒 | Se(s)+2H⁺+2e⁻ | ⇌ | H₂Se(g) | −0.11 | |
| 碳 | CO₂(g)+2H⁺+2e⁻ | ⇌ | CO(g)+H₂O | −0.11 | |
| 銅 | Cu(NH₃)₂⁺+e⁻ | ⇌ | Cu(s)+2NH₃(aq) | −0.1 | [37] |
| 锡 | SnO(s)+2H⁺+2e⁻ | ⇌ | Sn(s)+H₂O | −0.10 | |
| 锡 | SnO₂(s)+2H⁺+2e⁻ | ⇌ | SnO(s)+H₂O | −0.09 | |
| 钨 | WO₃(aq)+6H⁺+6e⁻ | ⇌ | W(s)+3H₂O | −0.09 | [3] |
| 磷 | P(白)+3H⁺+3e⁻ | ⇌ | PH₃(g) | −0.063 | [6] |
| 氫(氘) | 2D⁺+2e⁻ | ⇌ | D₂(g) | −0.044 | |
| 鐵 | Fe³⁺+3e⁻ | ⇌ | Fe(s) | −0.04 | [29] |
| 碳(甲酸) | HCO₂H(aq)+2H⁺+2e⁻ | ⇌ | HCHO(aq)+H₂O | −0.03 | |
| 氫 | 2H⁺+2e⁻ | ⇌ | H₂(g) | ≡0 | |
| 銀 | AgBr(s)+e⁻ | ⇌ | Ag(s)+Br⁻ | +0.07133 | [28] |
| 硫 | S₄O₆²⁻+2e⁻ | ⇌ | 2S₂O₃²⁻ | +0.08 | |
| 铁 | Fe₃O₄(s)+8H⁺+8e⁻ | ⇌ | 3Fe(s)+4H₂O | +0.085 | [9][10] |
| 氮 | N₂(g)+2H₂O+6H⁺+6e⁻ | ⇌ | 2NH₄OH(aq) | +0.092 | |
| 汞 | HgO(s)+H₂O+2e⁻ | ⇌ | Hg(l)+2OH⁻ | +0.0977 | |
| 銅 | Cu(NH₃)₄²⁺+e⁻ | ⇌ | Cu(NH₃)₂⁺+2NH₃ | +0.10 | [3] |
| 钌 | Ru(NH₃)₆³⁺+e⁻ | ⇌ | Ru(NH₃)₆²⁺ | +0.10 | [8] |
| 氮(肼) | N₂H₄(aq)+4H₂O+2e⁻ | ⇌ | 2NH₄⁺+4OH⁻ | +0.11 | [7] |
| 钼 | H₂MoO₄(aq)+6H⁺+6e⁻ | ⇌ | Mo(s)+4H₂O | +0.11 | |
| 鍺 | Ge⁴⁺+4e⁻ | ⇌ | Ge(s) | +0.12 | |
| 碳 | C(s)+4H⁺+4e⁻ | ⇌ | CH₄(g) | +0.13 | [3] |
| 碳 | HCHO(aq)+2H⁺+2e⁻ | ⇌ | CH₃OH(aq) | +0.13 | |
| 硫 | S(s)+2H⁺+2e⁻ | ⇌ | H₂S(g) | +0.14 | |
| 錫 | Sn⁴⁺+2e⁻ | ⇌ | Sn²⁺ | +0.15 | |
| 铜 | Cu²⁺+e⁻ | ⇌ | Cu⁺ | +0.159 | [3] |
| 硫 | HSO₄⁻+3H⁺+2e⁻ | ⇌ | SO₂(aq)+2H₂O | +0.16 | |
| 铀 | UO₂²⁺+e⁻ | ⇌ | UO₂⁺ | +0.163 | [8] |
| 硫 | SO₄²⁻+4H⁺+2e⁻ | ⇌ | SO₂(aq)+2H₂O | +0.17 | |
| 鈦 | TiO²⁺+2H⁺+e⁻ | ⇌ | Ti³⁺+H₂O | +0.19 | |
| 鉍 | Bi³⁺+2e⁻ | ⇌ | Bi⁺ | +0.2 | |
| 銻 | SbO⁺+2H⁺+3e⁻ | ⇌ | Sb(s)+H₂O | +0.20 | |
| 碳 | CO₂(g)+4H⁺+4e⁻ | ⇌ | C(s)+2H₂O | +0.205 | |
| 鐵 | 3Fe₂O₃(s)+2H⁺+2e⁻ | ⇌ | 2Fe₃O₄(s)+H₂O | +0.22 | :p.100 |
| 銀 | AgCl(s)+e⁻ | ⇌ | Ag(s)+Cl⁻ | +0.22233 | [28] |
| 砷 | H₃AsO₃(aq)+3H⁺+3e⁻ | ⇌ | As(s)+3H₂O | +0.24 | |
| 釕 | Ru³⁺(aq)+e⁻ | ⇌ | Ru²⁺(aq) | +0.249 | [38] |
| 鍺 | GeO(s)+2H⁺+2e⁻ | ⇌ | Ge(s)+H₂O | +0.26 | |
| 鈾 | UO₂⁺+4H⁺+e⁻ | ⇌ | U⁴⁺+2H₂O | +0.273 | [8] |
| 砹 | At₂+e⁻ | ⇌ | 2At⁻ | +0.3 | [12] |
| 錸 | Re³⁺+3e⁻ | ⇌ | Re(s) | +0.300 | |
| 鉍 | Bi³⁺+3e⁻ | ⇌ | Bi(s) | +0.32 | |
| 碳(氰) | 2HCNO+2H⁺+2e⁻ | ⇌ | (CN)₂+2H₂O | +0.330 | [39] |
| 钒 | VO²⁺+2H⁺+e⁻ | ⇌ | V³⁺+H₂O | +0.34 | |
| 銅 | Cu²⁺+2e⁻ | ⇌ | Cu(s) | +0.340 | [3] |
| 砈 | At⁺+2e⁻ | ⇌ | At⁻ | +0.36 | [40] |
| 鐵(鐵氰) | Fe(CN)₆³⁻+e⁻ | ⇌ | Fe(CN)₆⁴⁻ | +0.36 | |
| 碳(氰) | (CN)₂+2H⁺+2e⁻ | ⇌ | 2HCN | +0.373 | [41] |
| 鎝 | Tc²⁺+2e⁻ | ⇌ | Tc(s) | +0.40 | [12] |
| 氧 | O₂(g)+2H₂O+4e⁻ | ⇌ | 4OH⁻(aq) | +0.40 | [10] |
| 鉬 | H₂MoO₄+6H⁺+3e⁻ | ⇌ | Mo³⁺+2H₂O | +0.43 | |
| 釕 | Ru²⁺+2e⁻ | ⇌ | Ru(s) | +0.455 | [12] |
| 鉍 | Bi⁺+e⁻ | ⇌ | Bi(s) | +0.50 | |
| 碳 | CH₃OH(aq)+2H⁺+2e⁻ | ⇌ | CH₄(g)+H₂O | +0.50 | |
| 硫 | SO₂(aq)+4H⁺+4e⁻ | ⇌ | S(s)+2H₂O | +0.50 | |
| 銅 | Cu⁺+e⁻ | ⇌ | Cu(s) | +0.520 | [3] |
| 碳 | CO(g)+2H⁺+2e⁻ | ⇌ | C(s)+H₂O | +0.52 | |
| 碘 | I₃⁻+2e⁻ | ⇌ | 3I⁻ | +0.53 | [10] |
| 碘 | I₂(s)+2e⁻ | ⇌ | 2I⁻ | +0.54 | [10] |
| 金(金碘) | AuI₄⁻+3e⁻ | ⇌ | Au(s)+4I⁻ | +0.56 | |
| 砷 | H₃AsO₄(aq)+2H⁺+2e⁻ | ⇌ | H₃AsO₃(aq)+H₂O | +0.56 | |
| 金(金碘) | AuI₂⁻+e⁻ | ⇌ | Au(s)+2I⁻ | +0.58 | |
| 錳 | MnO₄⁻+2H₂O+3e⁻ | ⇌ | MnO₂(s)+4OH⁻ | +0.59 | |
| 銠 | Rh⁺+e⁻ | ⇌ | Rh(s) | +0.600 | [12] |
| 硫 | S₂O₃²⁻+6H⁺+4e⁻ | ⇌ | 2S(s)+3H₂O | +0.60 | |
| 鐵(二茂鐵) | Fc+e⁻ | ⇌ | Fc(s) | +0.641 | [42] |
| 银 | CH₃CO₂Ag+e⁻ | ⇌ | Ag+CH₃CO₂⁻ | +0.643 | [12] |
| 鉬 | H₂MoO₄(aq)+2H⁺+2e⁻ | ⇌ | MoO₂(s)+2H₂O | +0.65 | |
| 碳(苯醌) | 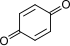 +2H⁺+2e⁻ +2H⁺+2e⁻ | ⇌ |  | +0.6992 | [28] |
| 氧 | O₂(g)+2H⁺+2e⁻ | ⇌ | H₂O₂(aq) | +0.70 | |
| 鉈 | Tl³⁺+3e⁻ | ⇌ | Tl(s) | +0.72 | |
| 鉑(鉑氯) | PtCl₆²⁻+2e⁻ | ⇌ | PtCl₄²⁻+2Cl⁻ | +0.726 | [8] |
| 鐵 | Fe₂O₃(s)+6H⁺+2e⁻ | ⇌ | 2Fe²⁺+3H₂O | +0.728 | :p.100 |
| 硒 | H₂SeO₃(aq)+4H⁺+4e⁻ | ⇌ | Se(s)+3H₂O | +0.74 | |
| 砈 | AtO⁺+2H⁺+2e⁻ | ⇌ | At⁺+H₂O | +0.74 | [43] |
| 銠 | Rh³⁺+3e⁻ | ⇌ | Rh(s) | +0.758 | [12] |
| 鉑(鉑氯) | PtCl₄²⁻+2e⁻ | ⇌ | Pt(s)+4Cl⁻ | +0.758 | [8] |
| 釙 | Po⁴⁺+4e⁻ | ⇌ | Po | +0.76 | [44] |
| 硫 | (SCN)₂+2e⁻ | ⇌ | 2SCN⁻ | +0.77 | [44] |
| 鐵 | Fe³⁺+e⁻ | ⇌ | Fe²⁺ | +0.77 | |
| 銀 | Ag⁺+e⁻ | ⇌ | Ag(s) | +0.7996 | [6] |
| 汞 | Hg₂²⁺+2e⁻ | ⇌ | 2Hg(l) | +0.80 | |
| 氮(硝) | NO₃⁻(aq)+2H⁺+e⁻ | ⇌ | NO₂(g)+H₂O | +0.80 | |
| 鐵 | FeO₄²⁻+5H₂O+6e⁻ | ⇌ | Fe₂O₃(s)+10OH⁻ | +0.81 | [29] |
| 金(金溴) | AuBr₄⁻+3e⁻ | ⇌ | Au(s)+4Br⁻ | +0.85 | |
| 汞 | Hg²⁺+2e⁻ | ⇌ | Hg(l) | +0.85 | |
| 銥 | IrCl₆²⁻+e⁻ | ⇌ | IrCl₆³⁻ | +0.87 | [45] |
| 錳 | MnO₄⁻+H⁺+e⁻ | ⇌ | HMnO₄⁻ | +0.90 | |
| 釙 | Po⁴⁺+2e⁻ | ⇌ | Po²⁺ | +0.9 | [46] |
| 汞 | 2Hg²⁺+2e⁻ | ⇌ | Hg₂²⁺ | +0.91 | [3] |
| 鈀 | Pd²⁺+2e⁻ | ⇌ | Pd(s) | +0.915 | [8] |
| 金(金氯) | AuCl₄⁻+3e⁻ | ⇌ | Au(s)+4Cl⁻ | +0.93 | |
| 錳 | MnO₂(s)+4H⁺+e⁻ | ⇌ | Mn³⁺+2H₂O | +0.95 | |
| 氮(硝) | NO₃⁻(aq)+4H⁺+3e⁻ | ⇌ | NO(g)+2H₂O(l) | +0.958 | [47] |
| 金(金溴) | AuBr₂⁻+e⁻ | ⇌ | Au(s)+2Br⁻ | +0.96 | |
| 鐵 | Fe₃O₄(s)+8H⁺+2e⁻ | ⇌ | 3Fe²⁺+4H₂O | +0.98 | :p.100 |
| 氙 | HXeO₆³⁻+2H₂O+2e⁻ | ⇌ | HXeO₄⁻+4OH⁻ | +0.99 | [48] |
| 氮(硝) | HNO₂+H⁺+e⁻ | ⇌ | NO(g)+H₂O | +0.996 | |
| 釩 | VO₂⁺(aq)+2H⁺+e⁻ | ⇌ | VO²⁺(aq)+H₂O | +1 | [49] |
| 砈 | HAtO+H⁺+e⁻ | ⇌ | At+H₂O | +1.0 | [50] |
| 碲 | H₆TeO₆(aq)+2H⁺+2e⁻ | ⇌ | TeO₂(s)+4H₂O | +1.02 | [51] |
| 溴 | Br₂(l)+2e⁻ | ⇌ | 2Br⁻ | +1.065 | |
| 溴 | Br₂(aq)+2e⁻ | ⇌ | 2Br⁻ | +1.087 | [10] |
| 氮(硝) | NO₂(g)+H⁺+e⁻ | ⇌ | HNO₂ | +1.093 | |
| 銅 | Cu²⁺+2CN⁻+e⁻ | ⇌ | Cu(CN)₂⁻ | +1.12 | [52] |
| 釕 | RuO₂+4H⁺+2e⁻ | ⇌ | Ru²⁺(aq)+2H₂O | +1.120 | [53] |
| 碘 | IO₃⁻+5H⁺+4e⁻ | ⇌ | HIO(aq)+2H₂O | +1.13 | |
| 金(金氯) | AuCl₂⁻+e⁻ | ⇌ | Au(s)+2Cl⁻ | +1.15 | |
| 硒 | HSeO₄⁻+3H⁺+2e⁻ | ⇌ | H₂SeO₃(aq)+H₂O | +1.15 | |
| 銥 | Ir³⁺+3e⁻ | ⇌ | Ir(s) | +1.156 | [12] |
| 銀 | Ag₂O(s)+2H⁺+2e⁻ | ⇌ | 2Ag(s)+H₂O | +1.17 | |
| 氯 | ClO₃⁻+2H⁺+e⁻ | ⇌ | ClO₂(g)+H₂O | +1.18 | |
| 氙 | HXeO₆³⁻+5H₂O+8e⁻ | ⇌ | Xe(g)+11OH⁻ | +1.18 | [48] |
| 铂 | Pt²⁺+2e⁻ | ⇌ | Pt(s) | +1.188 | [8] |
| 氯 | ClO₂(g)+H⁺+e⁻ | ⇌ | HClO₂(aq) | +1.19 | |
| 碘 | 2IO₃⁻+12H⁺+10e⁻ | ⇌ | I₂(s)+6H₂O | +1.20 | |
| 氯 | ClO₄⁻+2H⁺+2e⁻ | ⇌ | ClO₃⁻+H₂O | +1.20 | |
| 氧 | O₂(g)+4H⁺+4e⁻ | ⇌ | 2H₂O | +1.229 | [10] |
| 錳 | MnO₂(s)+4H⁺+2e⁻ | ⇌ | Mn²⁺+2H₂O | +1.23 | |
| 釕 | Ru(bipy)₃³⁺+e⁻ | ⇌ | Ru(bipy)₃²⁺ | +1.24 | [54] |
| 氙 | HXeO₄⁻+3H₂O+6e⁻ | ⇌ | Xe(g)+7OH⁻ | +1.24 | [48] |
| 鉈 | Tl³⁺+2e⁻ | ⇌ | Tl⁺ | +1.25 | |
| 鉻 | Cr₂O₇²⁻+14H⁺+6e⁻ | ⇌ | 2Cr³⁺+7H₂O | +1.33 | |
| 氯 | Cl₂(g)+2e⁻ | ⇌ | 2Cl⁻ | +1.36 | [10] |
| 釕 | RuO₄⁻(aq)+8H⁺+5e⁻ | ⇌ | Ru²⁺(aq)+4H₂O | +1.368 | [55] |
| 釕 | RuO₄+4H⁺+4e⁻ | ⇌ | RuO₂+2H₂O | +1.387 | [55] |
| 鈷 | CoO₂(s)+4H⁺+e⁻ | ⇌ | Co³⁺+2H₂O | +1.42 | |
| 氮(肼) | 2NH₃OH⁺+H⁺+2e⁻ | ⇌ | N₂H₅⁺+2H₂O | +1.42 | [7] |
| 碘 | 2HIO(aq)+2H⁺+2e⁻ | ⇌ | I₂(s)+2H₂O | +1.44 | |
| 鈰 | Ce⁴⁺+e⁻ | ⇌ | Ce³⁺ | +1.44 | |
| 溴 | BrO₃⁻+5H⁺+4e⁻ | ⇌ | HBrO(aq)+2H₂O | +1.45 | |
| 铅 | β-PbO₂(s)+4H⁺+2e⁻ | ⇌ | Pb²⁺+2H₂O | +1.460 | [3] |
| 铅 | α-PbO₂(s)+4H⁺+2e⁻ | ⇌ | Pb²⁺+2H₂O | +1.468 | [3] |
| 溴 | 2BrO₃⁻+12H⁺+10e⁻ | ⇌ | Br₂(l)+6H₂O | +1.48 | |
| 氯 | 2ClO₃⁻+12H⁺+10e⁻ | ⇌ | Cl₂(g)+6H₂O | +1.49 | |
| 氯 | HClO(aq)+H⁺+2e⁻ | ⇌ | Cl⁻(aq)+H₂O | +1.49 | [56] |
| 氧(超氧) | HO₂+H⁺+e⁻ | ⇌ | H₂O₂ | +1.495 | [12] |
| 砈 | HAtO₃+4H⁺+4e⁻ | ⇌ | HAtO+2H₂O | +1.5 | [57] |
| 錳 | MnO₄⁻+8H⁺+5e⁻ | ⇌ | Mn²⁺+4H₂O | +1.51 | |
| HO₂•+H⁺+e⁻ | ⇌ | H₂O₂(aq) | +1.51 | ||
| 金 | Au³⁺+3e⁻ | ⇌ | Au(s) | +1.52 | |
| 釕 | RuO₄²⁻(aq)+8H⁺+4e⁻ | ⇌ | Ru²⁺(aq)+4H₂O | +1.563 | [58] |
| 鎳 | NiO₂(s)+4H⁺+2e⁻ | ⇌ | Ni²⁺+2OH⁻ | +1.59 | |
| 氯 | 2HClO(aq)+2H⁺+2e⁻ | ⇌ | Cl₂(g)+2H₂O | +1.63 | |
| 碘 | IO₄⁻+2H⁺+2e⁻ | ⇌ | IO₃⁻+H₂O | +1.64 | [59] |
| 銀 | Ag₂O₃(s)+6H⁺+4e⁻ | ⇌ | 2Ag⁺+3H₂O | +1.67 | |
| 氯 | HClO₂(aq)+2H⁺+2e⁻ | ⇌ | HClO(aq)+H₂O | +1.67 | |
| 鉛 | Pb⁴⁺+2e⁻ | ⇌ | Pb²⁺ | +1.69 | [3] |
| 錳 | MnO₄⁻+4H⁺+3e⁻ | ⇌ | MnO₂(s)+2H₂O | +1.70 | |
| 銀 | AgO(s)+2H⁺+e⁻ | ⇌ | Ag⁺+H₂O | +1.77 | |
| 氧(過氧) | H₂O₂(aq)+2H⁺+2e⁻ | ⇌ | 2H₂O | +1.776 | |
| 鈷 | Co³⁺+e⁻ | ⇌ | Co²⁺ | +1.82 | |
| 金 | Au⁺+e⁻ | ⇌ | Au(s) | +1.83 | [3] |
| 溴 | BrO₄⁻+2H⁺+2e⁻ | ⇌ | BrO₃⁻+H₂O | +1.85 | |
| 銀 | Ag²⁺+e⁻ | ⇌ | Ag⁺ | +1.98 | [3] |
| 氧(過氧) | S₂O₈²⁻+2e⁻ | ⇌ | 2SO₄²⁻ | +2.07 | |
| 氧 | O₃(g)+2H⁺+2e⁻ | ⇌ | O₂(g)+H₂O | +2.075 | [8] |
| 錳 | HMnO₄⁻+3H⁺+2e⁻ | ⇌ | MnO₂(s)+2H₂O | +2.09 | |
| 氙 | XeO₃(aq)+6H⁺+6e⁻ | ⇌ | Xe(g)+3H₂O | +2.12 | [48] |
| 氧(氟氧) | OF₂+2H⁺+4e⁻ | ⇌ | 2F⁻+H₂O | +2.153 | [12] |
| 氙 | H₄XeO₆(aq)+8H⁺+8e⁻ | ⇌ | Xe(g)+6H₂O | +2.18 | [48] |
| 鐵 | FeO₄²⁻+8H⁺+3e⁻ | ⇌ | Fe³⁺+4H₂O | +2.20 | [60] |
| 氙 | XeF₂(aq)+2H⁺+2e⁻ | ⇌ | Xe(g)+2HF(aq) | +2.32 | [48] |
| 氙 | H₄XeO₆(aq)+2H⁺+2e⁻ | ⇌ | XeO₃(aq)+H₂O | +2.42 | [48] |
| 氟 | F₂(g)+2e⁻ | ⇌ | 2F⁻ | +2.87 | [3][10] |
| Cm | Cm⁴⁺+e⁻ | ⇌ | Cm³⁺ | +3.0 | [61] |
| 氟 | F₂(g)+2H⁺+2e⁻ | ⇌ | 2HF(aq) | +3.05 | [3] |
| 鋱 | Tb⁴⁺+e⁻ | ⇌ | Tb³⁺ | +3.05 | [12] |
| Pr | Pr⁴⁺+e⁻ | ⇌ | Pr³⁺ | +3.2 | [62] |
| 氪 | KrF₂(aq)+2e⁻ | ⇌ | Kr(g)+2F⁻(aq) | +3.27 | [63] |
参见编辑
参考资料编辑
- ^ R.W. Bosch, D.Feron, and J.P. Celis, "Electrochemistry in Light Water Reactors", CRC Press, 2007.
- ^ 2.0 2.1 2.2 2.3 2.4 2.5 Milazzo, G., Caroli, S., and Sharma, V. K. (1978). Tables of Standard Electrode Potentials (Wiley, Chichester).
- ^ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 3.14 3.15 3.16 3.17 3.18 3.19 Bard, A. J., Parsons, R., and Jordan, J. (1985). Standard Potentials in Aqueous Solutions (Marcel Dekker, New York).
- ^ 4.0 4.1 4.2 4.3 Bratsch, S. G. (1989). Journal of Physical Chemistry Reference Data Vol. 18, pp. 1–21. 引证错误:带有name属性“Bra”的
<ref>标签用不同内容定义了多次 - ^ 5.0 5.1 Vanýsek, Petr (2006). "Electrochemical Series," in Handbook of Chemistry and Physics: 87th Edition (页面存档备份,存于互联网档案馆) (Chemical Rubber Company). 引证错误:带有name属性“Van”的
<ref>标签用不同内容定义了多次 - ^ 6.00 6.01 6.02 6.03 6.04 6.05 6.06 6.07 6.08 6.09 6.10 6.11 6.12 6.13 6.14 6.15 6.16 6.17 6.18 6.19 6.20 6.21 6.22 6.23 6.24 6.25 6.26 6.27 6.28 6.29 6.30 Vanýsek, Petr (2007). “Electrochemical Series” (页面存档备份,存于互联网档案馆), in Handbook of Chemistry and Physics: 88th Edition (页面存档备份,存于互联网档案馆) (Chemical Rubber Company). 引证错误:带有name属性“van88”的
<ref>标签用不同内容定义了多次 - ^ 7.0 7.1 7.2 7.3 7.4 Greenwood, N. N.; Earnshaw, A. Chemistry of the Elements 2nd. Oxford:Butterworth-Heinemann. 1997. ISBN 0-7506-3365-4.
- ^ 8.00 8.01 8.02 8.03 8.04 8.05 8.06 8.07 8.08 8.09 8.10 8.11 Bard, A.J., Faulkner, L.R.(2001). Electrochemical Methods. Fundamentals and Applications, 2nd edition (John Wiley and Sons Inc).
- ^ 9.0 9.1 Marcel Pourbaix (1966). Atlas of Electrochemical Equilibria in Aqueous Solutions (NACE International, Houston, Texas; Cebelcor, Brussels).
- ^ 10.00 10.01 10.02 10.03 10.04 10.05 10.06 10.07 10.08 10.09 10.10 10.11 10.12 10.13 10.14 10.15 Peter Atkins (1997). Physical Chemistry, 6th edition (W.H. Freeman and Company, New York).
- ^ 11.0 11.1 11.2 Ca Sr Ba一價[11]與兩價間的標準電極電勢正好有規律關係,因此可以估計近似值
- ^ 12.00 12.01 12.02 12.03 12.04 12.05 12.06 12.07 12.08 12.09 12.10 12.11 12.12 12.13 12.14 12.15 12.16 12.17 12.18 12.19 12.20 12.21 12.22 12.23 12.24 12.25 12.26 12.27 12.28 12.29 12.30 12.31 12.32 12.33 12.34 Standard Redox Potential Table. [2012-01-14]. (原始内容存档于2021-02-06).
- ^ 13.00 13.01 13.02 13.03 13.04 13.05 13.06 13.07 13.08 13.09 13.10 13.11 13.12 13.13 13.14 13.15 13.16 13.17 13.18 13.19 13.20 13.21 13.22 13.23 13.24 13.25 13.26 13.27 13.28 13.29 13.30 13.31 13.32 13.33 13.34 13.35 13.36 Lide, David R. (编), CRC Handbook of Chemistry and Physics 87th, Boca Raton, FL: CRC Press, 2006, ISBN 0-8493-0487-3
- ^ Greenwood and Earnshaw, p. 1263
- ^ Standard Redox Potential Table. [2012-01-14]. (原始内容存档于2021-02-06).
- ^ Standard Redox Potential Table. [2012-01-14]. (原始内容存档于2021-02-06).
- ^ Vanýsek, Petr (2007). “Electrochemical Series” (页面存档备份,存于互联网档案馆), in Handbook of Chemistry and Physics: 88th Edition (页面存档备份,存于互联网档案馆) (Chemical Rubber Company).
- ^ 引证错误:没有为名为
van92的参考文献提供内容 - ^ David R. Lide, ed., CRC Handbook of Chemistry and Physics, Internet Version 2005, http://www.hbcpnetbase.com 互联网档案馆的存檔,存档日期2017-07-24., CRC Press, Boca Raton, FL, 2005.
- ^ Lide, David R. (编), CRC Handbook of Chemistry and Physics 87th, Boca Raton, FL: CRC Press, 2006, ISBN 0-8493-0487-3
- ^ Lide, David R. (编), CRC Handbook of Chemistry and Physics 87th, Boca Raton, FL: CRC Press, 2006, ISBN 0-8493-0487-3
- ^ Lide, David R. (编), CRC Handbook of Chemistry and Physics 87th, Boca Raton, FL: CRC Press, 2006, ISBN 0-8493-0487-3
- ^ Lide, David R. (编), CRC Handbook of Chemistry and Physics 87th, Boca Raton, FL: CRC Press, 2006, ISBN 0-8493-0487-3
- ^ 24.0 24.1 Lide, David R. (编), CRC Handbook of Chemistry and Physics 87th, Boca Raton, FL: CRC Press, 2006, ISBN 0-8493-0487-3
- ^ Ti Zr Hf 的標準電極電勢變化較規律,因此可估計 Rf的標準電極電勢
- ^ Gordon Aylward & Tristan Findlay (2008). "SI Chemical Data", 6th edition (John Wiley & Sons, Australia), ISBN 9780470816387.
- ^ List of carbon reactivity series (PDF). web.anl.gov. [2013-06-06]. (原始内容存档 (PDF)于2017-04-28). 可知碳之活性,九年義務教育課本《化學》九年級第一學期,上海教育出版社,2007年8月第2版,ISBN 978-7-5320-8481-4 第109、112頁、MSDS of carbon. [2013-06-06]. (原始内容存档于2016-03-05).根據碳的相關安全資料,可之其活性範圍,推之
- ^ 28.0 28.1 28.2 28.3 28.4 28.5 28.6 Vanýsek, Petr (2007). “Electrochemical Series”, in Handbook of Chemistry and Physics: 88th Edition (Chemical Rubber Company).
- ^ 29.0 29.1 29.2 29.3 29.4 WebElements Periodic Table of the Elements | Iron | compounds information. [2012-01-14]. (原始内容存档于2021-01-18).
- ^ Lide, David R. (编), CRC Handbook of Chemistry and Physics 87th, Boca Raton, FL: CRC Press, 2006, ISBN 0-8493-0487-3
- ^ 31.0 31.1 由−0.454和(2×−0.499+−0.508)÷3=−0.502推算出。
- ^ Lide, David R. (编), CRC Handbook of Chemistry and Physics 87th, Boca Raton, FL: CRC Press, 2006, ISBN 0-8493-0487-3
- ^ Bard, A. J., Parsons, R., and Jordan, J. (1985). Standard Potentials in Aqueous Solutions (Marcel Dekker, New York).
- ^ “Glyoxal Bisulfite” (页面存档备份,存于互联网档案馆), Organic Syntheses, Collected Volume 3, p.438 (1955).
- ^ Huang, Haiyan; Shuning Wang; Johanna Moll; Rudolf K. Thauer. Electron Bifurcation Involved in the Energy Metabolism of the Acetogenic Bacterium Moorella thermoacetica Growing on Glucose or H2 plus CO2. Journal of Bacteriology. 2012-07-15, 194 (14): 3689–3699 [2013-09-10]. ISSN 0021-9193. doi:10.1128/JB.00385-12. (原始内容存档于2020-12-13).
- ^ Petr Vanysek. Electrochemical series (PDF). depa.fquim.unam.mx. (原始内容存档 (PDF)于2022-10-16).
- ^ Bard, A. J., Parsons, R., and Jordan, J. (1985). Standard Potentials in Aqueous Solutions (Marcel Dekker, New York).
- ^ Greenwood and Earnshaw, p. 1077
- ^ Petr Vanysek. Electrochemical series (PDF). depa.fquim.unam.mx. (原始内容存档 (PDF)于2022-10-16).
- ^ {{cite journal | last=Champion | first=J. | last2=Alliot | first2=C. | last3=Renault | first3=E. | last4=Mokili | first4=B. M. | last5=Chérel | first5=M. | last6=Galland | first6=N. | last7=Montavon | first7=G. | title=Astatine Standard Redox Potentials and Speciation in Acidic Medium | journal=The Journal of Physical Chemistry A | publisher=American Chemical Society (ACS) | volume=114 | issue=1 | date=2009-12-16 | issn=1089-5639 | doi=10.1021/jp9077008 | pages=576–58₂]]
- ^ Petr Vanysek. Electrochemical series (PDF). depa.fquim.unam.mx. (原始内容存档 (PDF)于2022-10-16).
- ^ Connelly, Neil G.; Geiger, William E. Chemical Redox Agents for Organometallic Chemistry. Chemical Reviews. 1 January 1996, 96 (2): 877–910. PMID 11848774. doi:10.1021/cr940053x.
- ^ {{cite journal | last=Champion | first=J. | last2=Alliot | first2=C. | last3=Renault | first3=E. | last4=Mokili | first4=B. M. | last5=Chérel | first5=M. | last6=Galland | first6=N. | last7=Montavon | first7=G. | title=Astatine Standard Redox Potentials and Speciation in Acidic Medium | journal=The Journal of Physical Chemistry A | publisher=American Chemical Society (ACS) | volume=114 | issue=1 | date=2009-12-16 | issn=1089-5639 | doi=10.1021/jp9077008 | pages=576–58₂]]
- ^ 44.0 44.1 Petr Vanysek. Electrochemical series (PDF). depa.fquim.unam.mx. (原始内容存档 (PDF)于2022-10-16).
- ^ {{cite book |last= Atkins |first=Peter |title= Inorganic Chemistry |edition=5th |year=2010 |publisher=W. H. Freeman |isbn= 978-1-42-921820-7 |pages=15₃]]
- ^ Petr Vanysek. Electrochemical series (PDF). depa.fquim.unam.mx. (原始内容存档 (PDF)于2022-10-16).
- ^ Peter Atkins (1997). Physical Chemistry, 6th edition (W.H. Freeman and Company, New York).
- ^ 48.0 48.1 48.2 48.3 48.4 48.5 48.6 WebElements Periodic Table of the Elements | Xenon | compounds information. [2012-01-14]. (原始内容存档于2021-03-22).
- ^ Cotton, F. Albert; Wilkinson, Geoffrey; Murillo, Carlos A.; Bochmann, Manfred, Advanced Inorganic Chemistry 6th, New York: Wiley-Interscience, 1999, ISBN 0-471-19957-5
- ^ Lavrukhina, Avgusta Konstantinovna; Pozdni︠a︡kov, Aleksandr Aleksandrovich. Analytical chemistry of technetium, promethium, astatine and francium. Ann Arbor: Ann Arbor-Humphrey Science Publishers. 1970: 237. ISBN 0-250-39923-7. OCLC 186926.
- ^ Cotton, F. Albert; Wilkinson, Geoffrey; Murillo, Carlos A.; Bochmann, Manfred, Advanced Inorganic Chemistry 6th, New York: Wiley-Interscience, 1999, ISBN 0-471-19957-5
- ^ Bard, A. J., Parsons, R., and Jordan, J. (1985). Standard Potentials in Aqueous Solutions (Marcel Dekker, New York).
- ^ Greenwood and Earnshaw, p. 1077
- ^ Lide, David R. (编), CRC Handbook of Chemistry and Physics 87th, Boca Raton, FL: CRC Press, 2006, ISBN 0-8493-0487-3
- ^ 55.0 55.1 Greenwood and Earnshaw, p. 1077
- ^ Lide, David R. (编), CRC Handbook of Chemistry and Physics 87th, Boca Raton, FL: CRC Press, 2006, ISBN 0-8493-0487-3
- ^ Lavrukhina, Avgusta Konstantinovna; Pozdni︠a︡kov, Aleksandr Aleksandrovich. Analytical chemistry of technetium, promethium, astatine and francium. Ann Arbor: Ann Arbor-Humphrey Science Publishers. 1970: 237. ISBN 0-250-39923-7. OCLC 186926.
- ^ Greenwood and Earnshaw, p. 1077
- ^ Appelman, Evan H. Nonexistent compounds. Two case histories. Accounts of Chemical Research (American Chemical Society (ACS)). 1973-04-01, 6 (4): 113–117. ISSN 0001-4842. doi:10.1021/ar50064a001.
- ^ Redox Reactions, Western Oregon University website. [2012-01-15]. (原始内容存档于2019-08-30).
- ^ Petr Vanysek. Electrochemical series (PDF). depa.fquim.unam.mx. (原始内容存档 (PDF)于2022-10-16).
- ^ Petr Vanysek. Electrochemical series (PDF). depa.fquim.unam.mx. (原始内容存档 (PDF)于2022-10-16).
- ^ Leszczyński, P.J.; Grochala, W. Strong Cationic Oxidizers: Thermal Decomposition, Electronic Structure and Magnetism of Their Compounds (PDF). Acta Chim. Slov. 2013, 60 (3): 455–470. PMID 24169699. (原始内容存档 (PDF)于2022-10-09).







